Quratis
We hope to become a company with a social impact that leads the human public health.
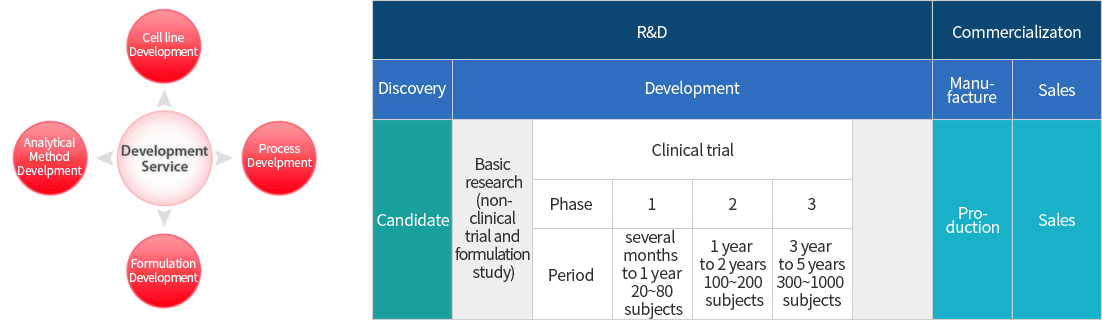
CMO & CDMO
Contract Development and Manufacturing Organization
- HOME
- Business
- CMO & CDMO
(Clinical trial) GMP Biomanufacturing
-
→ Recombinant DNA based cGMP Biomanufacturing Facility and Wide Work Experience
- ∙ cGMP Compliant Manufacturing Facility for (clinical trial) Biologics (Expected to be certified in 2020)
- ∙ Developing Reproducible Manufacturing Process from Small Scale to Commercial Scale
- ∙ cGMP Manufacturing and Process Technology for (clinical trial) Biologics based on Microbial Fermentation
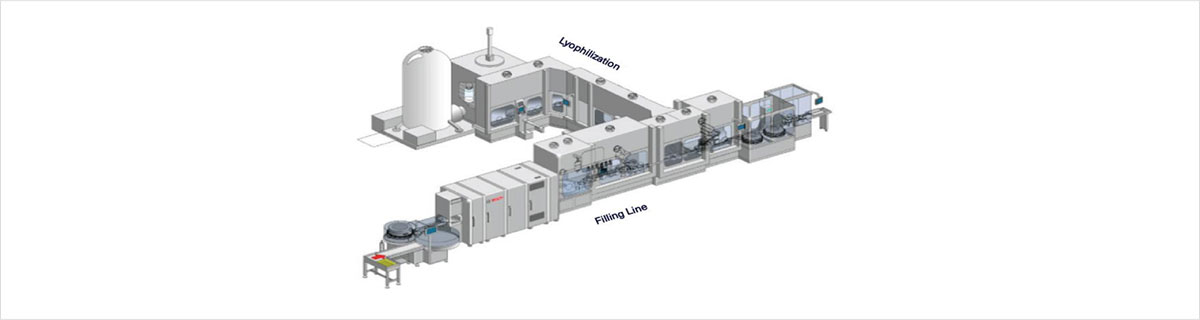
- ∙ cGMP Manufacturing and Process Technology for Parenterals(Liquid, Freeze-dry)
- ∙ QA Experts Experienced with Various Inspections from Domestic and Overseas Regulatory Authority
- ∙ Quality Program (Quality system, Facilities & Equipment, Materials, Productions, Packaging & Labeling, Laboratory control) covers cGMP, EU-GMP, KGMP and ICH guidelines
- ∙ Well Defined Manufacturing Environment: Grade A/B (Sterile filling and supporting area), Grade C/D (microbial control zone), CNC (Controlled not classified)
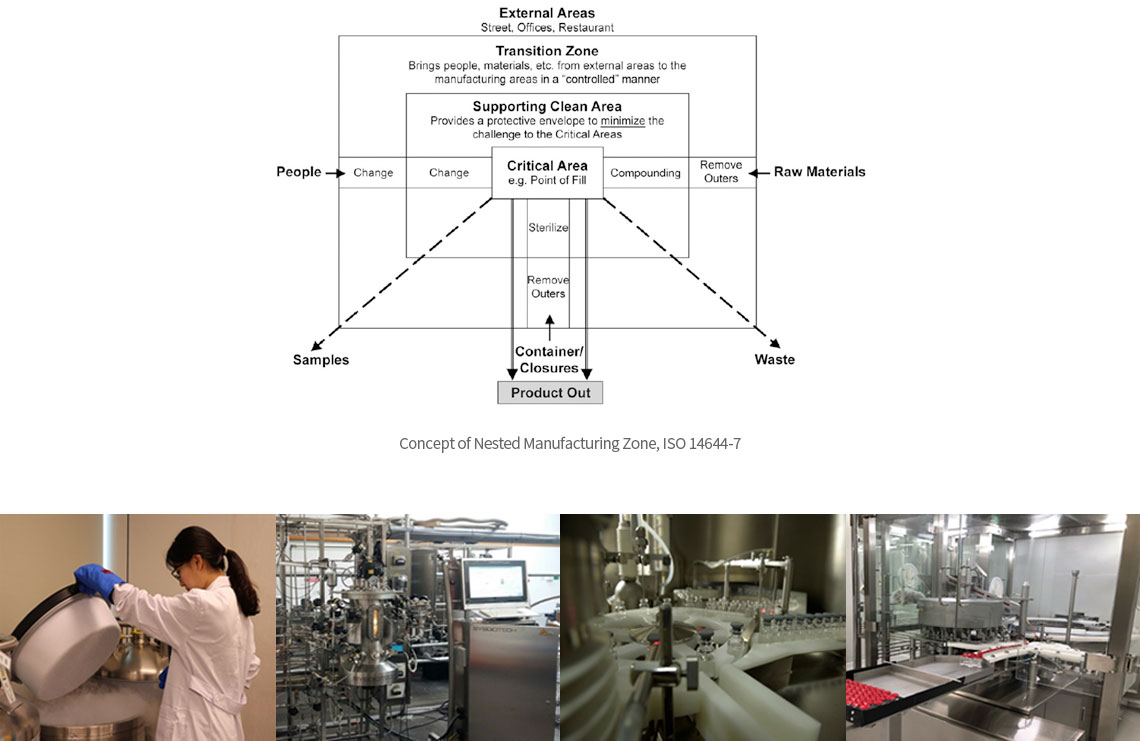
∙ Recombinant DDA based Biologics Develop Process
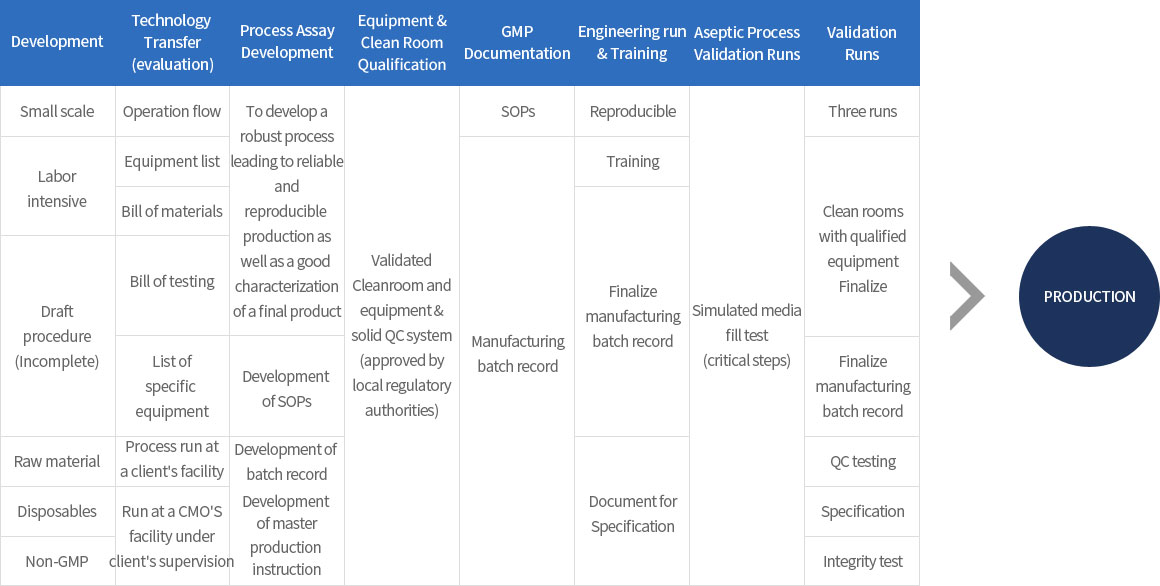
∙ GMP manufacturing and validation service for Drug Substance and Drug Product of (clinical trial) Biologics using recombinant DNA technology
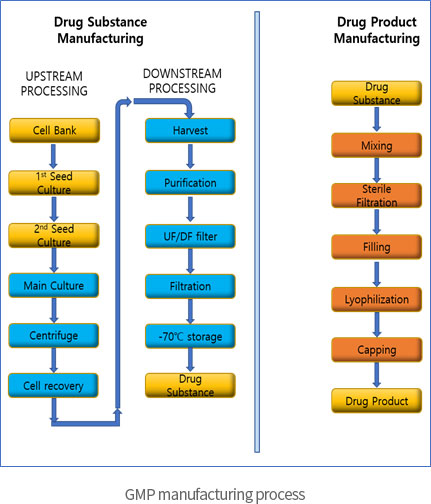
Non-GMP manufacturing for non-clinical trial
-
→ To provide R&D and non-clinical trial drug production service from early stage development for DS and DP of microbial fermentation based recombinant biologics
- ∙ Fermentation and Purification Process for Recombination (E.coli) Cell Line
- ∙ Liquid Injection and Freeze-Drying Injection
- ∙ Analytical Method Development (Identity, Quantity, Potency, Impurities)

Customized Services
-
→ Customized Services including Manufacturing Process Development of biologics DS and DP
- ∙ Cell Line Screening for Recombinant DNA Biopharmaceuticals
- ∙ Development of Fermentation and Purification Condition Satisfied with Customer’s Requirements
- ∙ Optimization of Fermentation and Purification Process for Drug Substance
- ∙ Specialized to Validation and Regulatory Affairs Services
-
→ Fermentation process development
- ∙ Development and Improvement of Fermentation Condition
- ∙ Fermentation Process Development: Batch, Fed-batch, Seed Culture
- ∙ Basal Media and Feeding Media Screening
- ∙ Fermentation Process Optimization(Study regarding Process Parameters such as Feeding Strategy, Temperature, pH, Initial Cell Density, etc.)
- ∙ Setup for 50 L and 500 L Fermentation Process
-
→ Purification Process Development
- ∙ Process Improvement and Optimization
- ∙ Purification Process Development
- ∙ Type & Sizing test for UF/DF Membrane
- ∙ Setup for Purification Process
-
→ Scale-up Study
- ∙ 50 L/500 L Volume Process Setup and Analysis
- ∙ Technical Transfer of Manufacturing for (Non-) Clinical trial
-
→ Formulation Development
- ∙ Optimization on Product and Stability through Formulation Development for Protein and Excipients
- ∙ Formulation Development for Liquid Injection and Freeze-Drying Injection
-
→ Analytical Method Development and Support
- ∙ Analytical Method Development suitable for Each Product Characteristics(Identity, Quantity, Purity, Potency, Impurities, Endotoxin, Physico-chemical tests)
- ∙ Analytical Method Validation (Specificity, Accuracy, Precision, Detection Limit, Quantitation, Limit, Linearity, Range, Robustness)
- ∙ Stability tests
-
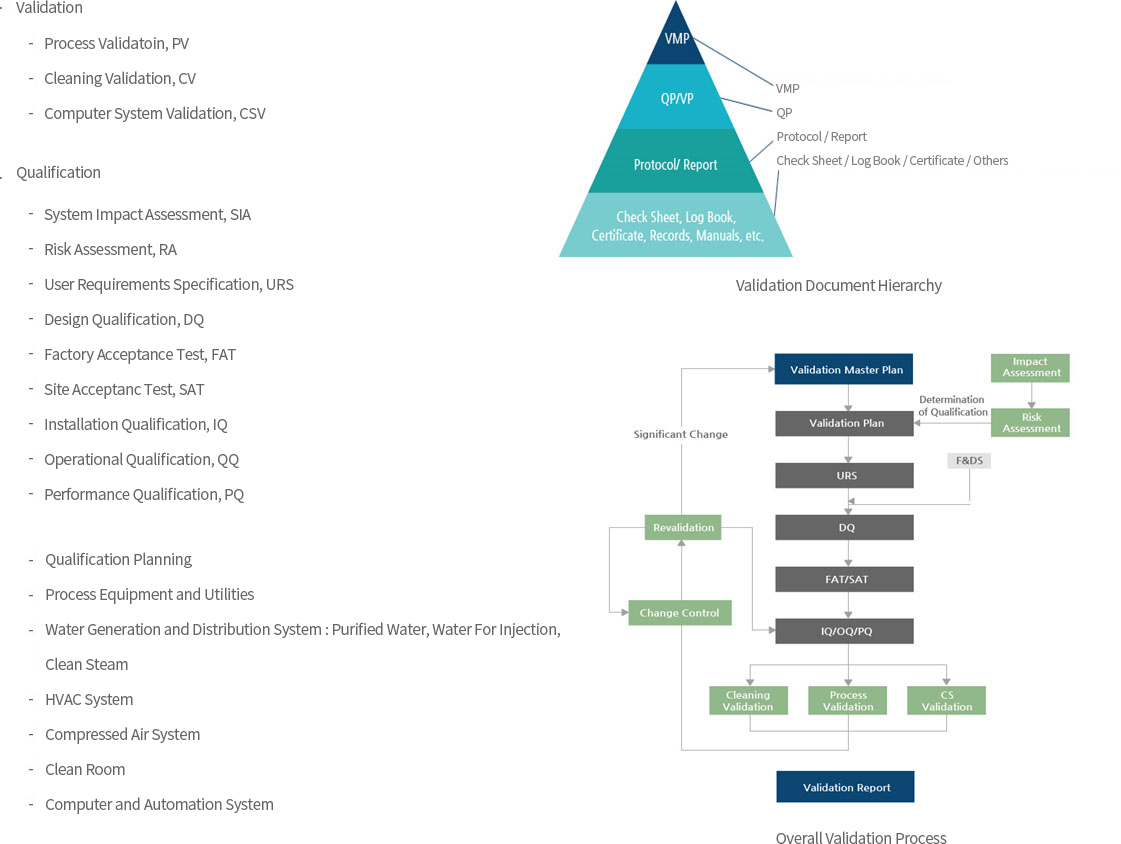
→ Validation
- ∙ To carry on Procedure on Validation and Qualification Guideline